Validation and Qualification in Laboratory Analytics
The qualification of devices and systems is an essential element of validation processes. As a manufacturer of laboratory instruments, we at A.KRÜSS meet the enormous demand for qualification services with a comprehensive concept.
Qualification: Our four-stage process
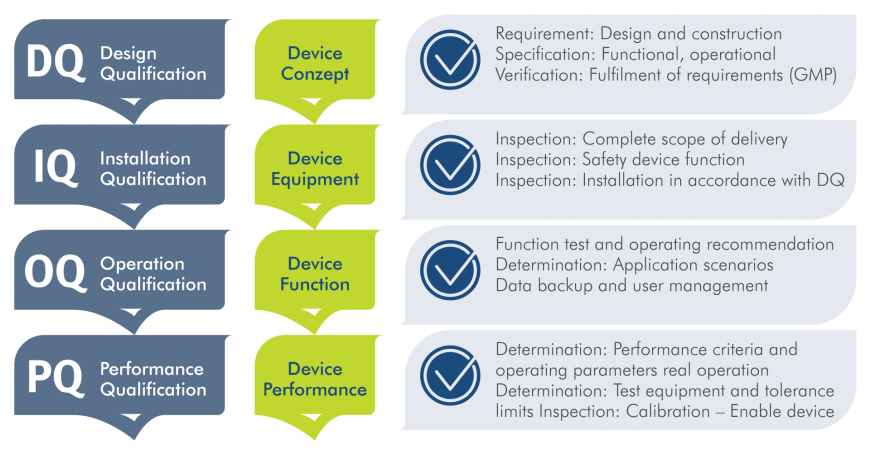
We work together with you, our customers, hand in hand and offer comprehensive support throughout the entire qualification process.
Qualification in accordance with valid guidelines, standards and laws
Our four-stage qualification is intended to save you costs, resources and time in the process. Our specialists can draw on many years of experience in the qualification of measuring instruments and are fully familiar with the current applicable regulatory requirements of the prevailing directives, standards and laws. Below is a selection of the requirements that are important to us:
- EC-GMP-Guidelines
- cGMP Rules for Medical Devices
- Good Automated Manufacturing Practice (GAMP) Guidelines
- FDA-compliant qualification of systems (21 CFR Part 11)
- Requirements of pharmacopoeias or USP
- DAkkS Guidelines
- Based on the requirements of DIN EN ISO 17025
A.KRÜSS qualification with expertise at your side
We bring along the knowledge about the devices, measurement technology and operation and integrate it into the manufacturing processes of our customers. Our number one priority: We want to ensure that you can carry out your quality control effectively and reliably. The devices that we optimally configure for you should give you confidence in the quality and significance of your daily analysis results.
- Qualification with A.KRÜSS is achieved by reducing the expenditure to a justifiable level, without mountains of paper, with easy-to-understand documents.
- The documents can be requested in advance from us for viewing.
- On site we work by using the necessary specialist tools and measurement equipment (traceable standards). We have an extensive expertise in test equipment management, as well as in hazard assessment and analysis of risks.
- We know that today’s digital devices are subject to stringent testing in terms of data integrity. This is why we initialise the proper device firmware configuration for you and the correct operating competence so that your software applications always fit perfectly.
- We work with electronic signatures, the uniqueness of the combination of user ID and password.
- The data documentation is flanked by a two-stage audit trail and audit trail log concept.
Two qualification models

We understand the different needs of our customers. Various qualification models exist for highly regulated areas and for laboratories with standard requirements:
Standard qualification
The standard qualification is a GMP / GLP compliant qualification of laboratory devices at our customers on site.
- Including all necessary IQ, OQ and PQ services in the working environment of our customers
- Including the creation of all necessary documents, test reports and certificates
- Including the preparation of all certified testing equipment and measurement, control and special tools required for qualification
- Including SOP support according to the requirements of the user and the measurement task
- Including extensive application-related training of the operating personnel
Standard qualification including PharmaKit
This is the recommended qualification variant for highly regulated, mostly pharmaceutical laboratories (rarely cosmetics and food). Based on the standard qualification, further requirements are covered, such as:
- Including all additional services and necessary documents that certify and assess conformity with the GAMP5 assessment, GMP, USP1058 and 21 CFR Part 11
- Including all necessary services and documents required for a comprehensive DQ, IQ, OQ, PQ qualification
- If necessary, this qualification contains a preliminary consultation and an examination, commenting and conformity assessment of the fixed written DQ requirements in your customer specification
- With all necessary documents for risk analysis, risk-based documentation or hazard assessment
- Including testing and documentation of software requirements via comprehensible checklist
- With extensive SOP support on site including documentation
- User training which, in addition to the measurement methodology of operation and cleaning, includes the security aspects of audit trail, user administration and data integrity
- Plus all services included in the standard license described above
If you require our personal assistance, please contact our Technical Service Center in Germany using our contact form or by telephone. We will be happy to assist you with your enquiry. Our Sales and Service Partner Network ensure optimal on site service, personalised advice and timely support outside of Germany.
Service hotline:
Technical Service Center – Germany
Phone: +49 – 40 – 514 317 – 0
Sales and Service Partner – Worldwide
Sales and Service Partner Network